Abstract
Introduction
Emicizumab is a therapeutic bispecific antibody being studied for the the prevention of bleeding in people with hemophilia A. HAVEN 1 and HAVEN 2 are multicenter, open-label phase 3 studies of subcutaneous administration of emicizumab in patients with hemophilia A with inhibitors; HAVEN 1 in adults and adolescents, and HAVEN 2 in children. Patients with planned surgeries, with the exception of minor procedures, were excluded from both studies. However, unplanned emergency surgeries, in addition to minor procedures, were performed in patients receiving emicizumab. Perioperative management was at the discretion of the investigators based on individual clinical assessment, without specific guidance (per protocol) on surgical management provided by the sponsor.
Methods
We describe the combined surgical experiences from HAVEN 1 and an interim analysis of HAVEN 2, specifically, the frequency of peri-operative bypassing agent (BPA) use and post-operative bleeding events in patients who were treated with emicizumab. Additionally, the type and frequency of surgical procedures and the numbers of surgical procedures associated with and without use of prophylactic BPAs are summarized. Frequency of post-operative bleeding, which were reported in the data as bleeding events resulting from a surgery or procedure, as well as the doses of peri-operative BPAs are also described. Bleeding events were defined as in HAVEN 1 (Oldenburg et al. NEJM 2017; July 10: epub).
Results
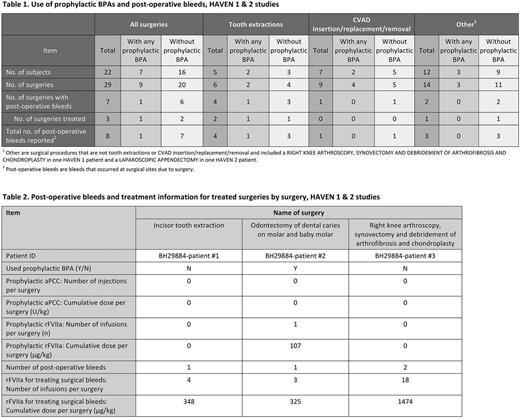
In HAVEN 1 and in the interim analysis of HAVEN 2, there were 22 patients who underwent a total of 29 surgical procedures (24 surgical procedures in 17 patients in HAVEN 1, and 5 surgical procedures in 5 patients in HAVEN 2). Twenty of these procedures (69%) were managed without prophylactic BPAs. Nine procedures (31%) were managed with prophylactic BPAs. Among the 29 surgeries, 6 were tooth extractions and 9 were central venous access device (CVAD)-related procedures (insertion/replacement/removal). Of the remaining 14 surgical procedures, 12 were minor and 2 were major: (1) right knee arthroscopy, synovectomy, debridement of arthrofibrosis, and chondroplasty in HAVEN 1, and (2) a laparoscopic appendectomy in HAVEN 2.
Among the 20 surgeries that were not managed with surgical prophylactic BPAs, 14 (70%) did not result in post-operative bleed(s), and 6 (30%) resulted in post-operative bleeds (2 treated with BPAs and 4 not treated with BPAs). The 2 treated post-operative bleeding events occurred with a tooth extraction and with a right knee arthroscopy, synovectomy, debridement of arthrofibrosis, and chondroplasty.
Among the 9 surgeries that were managed with surgical prophylactic BPA, 8 were managed with rFVIIa with mean dose (range): 152.81 µg/kg (86.54-254.72 µg/kg) and median number of injections of 1; 1 surgery used prophylactic aPCC (single dose of 49.78 U/kg). Eight (89%) of these surgeries did not result in post-operative bleeding, and 1 (11%) was a tooth extraction that resulted in a single treated post-operative bleeding event.
Anti-fibrinolytics were used in 4 of 29 surgical procedures, 3 of which were tooth extractions and 1 of which was a CVAD removal.
Among the 7 surgeries that resulted in post-operative bleeding, 3 surgeries were managed with rFVIIa and no surgery was managed with aPCC. BPA doses administered post-operatively in these surgeries are shown in Table 2.
Conclusions
In HAVEN 1 and an interim analysis of HAVEN 2,management of perioperative BPA use was at the discretion of the investigator. In the two studies combined, the majority (20/29; 69%) of surgeries performed were not managed with prophylactic BPAs, with no post-operative bleeding reported in the majority (14/20; 70%) of these surgeries. Among the 4 tooth extractions that were not managed with prophylactic BPAs, 3 resulted in post-operative bleeding, only 1 of which was treated with BPAs. Among the 5 CVAD-related procedures that were not managed with prophylactic BPAs, only 1 resulted in a post-operative bleed that was not treated with BPAs. In this analysis of patients with hemophilia A with inhibitors who underwent mostly minor surgical procedures while receiving emicizumab prophylaxis, the majority of patients did not receive pre-operative treatment with BPAs. Post-operative bleeding requiring additional BPA treatment seldom occurred.
Kruse-Jarres: Roche/Genentech: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; CSL Behring: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Shire: Consultancy, Honoraria; Grifols: Consultancy, Honoraria; Baxalta: Honoraria. Callaghan: CSL Behring: Membership on an entity's Board of Directors or advisory committees; Bayer HealthCare; Pfizer Inc.; Roche; Shire: Consultancy; Biogen: Membership on an entity's Board of Directors or advisory committees; Roche; Shire: Speakers Bureau; Global Blood Therapeutics: Other: Site PI; Grifols: Membership on an entity's Board of Directors or advisory committees; Alnylam Pharmaceuticals, Inc: Other: Owns stock, stock options, or bonds ; Novo Nordisk: Speakers Bureau; Baxalta: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche/Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Site PI, Speakers Bureau; Sancillio: Other: Site PI; Octapharma: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer Inc.: Membership on an entity's Board of Directors or advisory committees, Other: Site PI, Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees. Croteau: Aptevo, Baxalta/Shire, Bayer, CSL Behring, Genentech, Novo Nordisk, Octapharma, Pfizer: Consultancy; ATHN/Hemophilia of Georgia: Research Funding; Octapharma: Honoraria; Baxalta, Dimension Therapeutics, Genentech, Pfizer: Research Funding. Jimenez-Yuste: Novo Nordisk: Consultancy, Honoraria, Research Funding; Roche: Consultancy. Khoo: Novo Nordisk: Honoraria; Biogen: Honoraria; Baxalta/Shire: Honoraria. Liesner: Bio Products Laboratory: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; SOBI: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Baxalta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; NovoNordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; SOBI/Bioverativ: Research Funding, Speakers Bureau. Matsushita: Chugai: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Recht: Biogen: Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Kedrion: Membership on an entity's Board of Directors or advisory committees; NovoNordisk: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees, Research Funding. Young: Novo Nordisk: Consultancy; CSL Behring: Honoraria. Chang: Genentech: Employment, Equity Ownership. Dhalluin: F. Hoffmann-La Roche Ltd: Employment. Mu: Genentech, Inc.: Employment. Xu: Genentech: Employment. Devenport: Genentech, Inc.: Employment. Ko: Genentech, Inc.: Employment. Solari: Genentech, Inc.: Employment. Oldenburg: Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biotest: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Grifols: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Honoraria, Investigator Clinical Studies and Research Funding, Membership on an entity's Board of Directors or advisory committees, Research Funding; Baxter: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Baxalta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Investigator Clinical Studies and Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Chugai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Biogen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal